Contaminants of emerging concern

By L Kuskopf, M Sheehan, A Whelan.
First published in Water e-Journal Vol 5 No 3 2020.
Abstract
There are potentially many thousands of municipally derived contaminants of emerging concern (CECs) present in discharged wastewaters that may cause adverse effects in receiving aquatic environments. Wastewater authorities, therefore, may face the momentous task of investigating these compounds with little or no prior CEC data for their sewage treatment plants (STPs). Such is the case for the Cleveland Bay Sewage Treatment Plant (CBSTP).
To evaluate the potential environmental risk posed by municipal CECs, it is first necessary to understand which CECs are present in discharging wastewater, how they will be sampled and quantified, and which CECs are the most concerning regarding ecological risk.
A Sampling Analysis and Quality Plan (SAQP) is a valuable tool that can be used to critically review and detail the strategy that will be adopted to achieve these outcomes. As such, this paper describes the development of a SAQP for screening wastewaters released from the CBSTP into Cleveland Bay as part of a preliminary CEC assessment.
This paper describes the qualitative decision-making process employed to shortlist CECs into those perceived to pose the greatest ecological risk. In addition, the rationale and methods adopted to determine sampling locations and frequency are described.
The preliminary results of the analysis are subsequently used to assess the success or otherwise of the selection methodology, exploring the implications of these results.
Introduction
CECs may be defined as any natural, manufactured or manmade chemical seldom monitored in the environment that is suspected, or known, to cause adverse ecological effects. These include a wide array of pharmaceuticals, personal care products, food additives, illicit drugs, microplastics, nanomaterials, pesticides, flame retardants, plasticisers and other industrial chemicals.
Following a decade of advancement in analytical methods applied to characterise wastewater, researchers, water authorities and industry experts are becoming increasingly aware of the persistence of CECs through conventional STPs.
Per- and poly-fluoroalkyl substances (PFAS), for example, have drawn unprecedented international attention over the past few years [1] on account of the persistent, bio-accumulative and biomagnifying nature of these compounds [2].
Environmental harm is also feared following recent correlations of antibiotic concentrations to the presence of antibiotic resistant genes (ARGs) [3], considered to undermine the effectiveness of current and future antibiotics [4]. These discoveries, among others, have generated renewed concerns for receiving environments subject to effluent discharge.
Townsville City Council (TCC) owns and operates CBSTP, located on the coast of Townsville, Queensland. It represents the largest point source wastewater discharge in the reef region, treating an equivalent population of over 100,000 (about 25 ML/day).
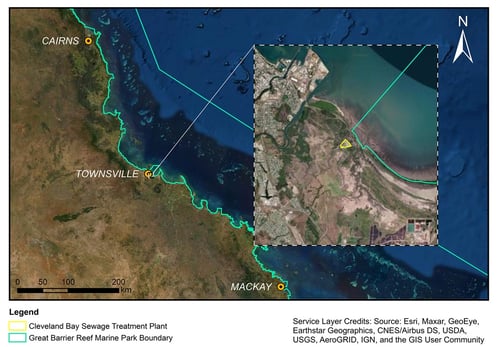
CBSTP’s effluent is discharged via a marine ocean outfall onto tidal mudflats in Cleveland Bay. The discharge point shown in Figure 1 lies within the jurisdiction of the Queensland State Marine Park (QSMP), and within the general use zone of Great Barrier Reef Marine Park Authority (GBRMP).
Located adjacent to the Great Barrier Reef (GBR), as shown in Figure 2, Cleveland Bay is recognised as holding important regional and national ecological value. For example, iconic marine wildlife protected under Commonwealth and state regulation including turtles and dugongs, frequent the bay [5, 6]. Protection of these and other unique ecological values is therefore of national importance.
Ecological risk assessment (ERA) is an integration of environmental toxicology, chemistry, ecology and hydrology [7]. Employed to quantify the probability of adverse ecological impacts, ERAs must consider not only exposure of an organism to a stressor (e.g. an emerging contaminant), but also the effect that stressor may have (accounting for its severity) [8, 9].
To commence an investigation into either of these pillars of risk assessment, it is first necessary to determine which stressors to investigate. For this reason, the first step of an ERA (commonly termed ‘problem formulation’) essentially encapsulates the entire process; specifically, CECs and their corresponding undesired ecological effects must be identified to structure the subsequent investigation [8].
Unfortunately, the nature of STP influent presents a host of opportunities for the introduction of CECs. In fact, hundreds of CECs are generally detectable at micro and nanogram concentrations in wastewater effluents [10]. This introduces significant uncertainty regarding which CECs should be the target of investigation.
There also remains a lot of uncertainty as to the fate of these chemicals in the receiving environment, increasing the challenge of prioritising CECs for an ERA.
TCC seeks to ascertain the potential ecological risk that CECs pose to the unique North Queensland ecology of Cleveland Bay. To facilitate this subsequent ERA, an initial screening program of CEC’s is required to determine which contaminants persist through CBSTP and may be useful in later receiving environmental assessments.
Given the many thousands of municipally derived CECs present in discharged wastewaters and the considerable analytical challenge and expense involved in their quantification, a means to refine and reduce this list is necessary for practicality. The intent is to ensure that those CECs considered most likely to be present and pose a risk to the receiving environment are captured in the SAQP.
It is widely acknowledged that the quality of any investigation is critically influenced by the care devoted to the selection of sample locations, frequency, collection procedures, and preservation prior to analysis. Failing to consider the limitations of adopted sampling methodologies can result in the unnecessary expenditure of resources to acquire biased, imprecise, and inaccurately measured CEC concentrations.
The literature surrounding CECs is constantly expanding, and so a review of the site selection rationale and collection procedures is presented here to inform those working ‘on the ground’ of the current state of knowledge and practice.
Overall, the methodologies described in this paper should be a valuable and practical guide to water authorities concerned with the quantification of ecological risk posed by CECs discharged in effluent.
Preliminary screening purpose
The intention of the preliminary screening program and development of a SAQP is principally to improve our understanding of CEC exposure from the point source of CBSTP’s outfall.
Despite the breadth of available literature on CEC concentrations representing different classes in wastewaters [11], a similarly diverse suite of CEC classes in the GBR have not been investigated [12-14]. Further, other investigation of multiple CEC classes for advanced STPs in Australia are uncommon.
As a membrane bioreactor (MBR) plant discharging directly adjacent to the GBR, CBSTP is uniquely positioned to provide insight into the success or otherwise of CEC removal.
SAQP objectives
It was considered that the SAQP described herein must:
- Quantify a sufficiently comprehensive array of CECs from different chemical classes at CBSTP to validate their presence / absence in wastewaters.
- Capture contaminants considered most likely to pose a risk to the receiving environment, based on current scientific knowledge.
- Employ a sampling rationale appropriate to the target contaminants, which delivers a suitable degree of accuracy and precision
- Utilise containers and preservation methods appropriate to the intended analysis.
- Consider quality assurance and control methods to evaluate the quality of reported data.
Methodology
Summarised in Figure 3 are the three preliminary project phases presented in this paper. The components within each phase are described in greater detail in this section.
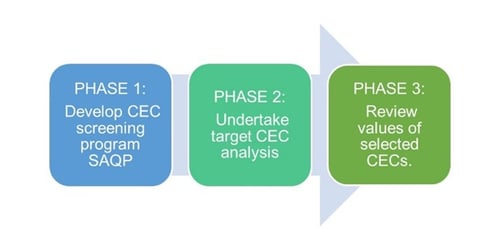 Figure 3: Project phases 1 to 3 comprising of CEC screening program development, analysis, and subsequent review
Figure 3: Project phases 1 to 3 comprising of CEC screening program development, analysis, and subsequent review
Phase 1 – Developing CEC screening program SAQP
The SAQP development procedure may be divided into two key steps, namely:
Step 1 – Develop a target CEC list
- Undertake a general review of CEC literature.
- Compile factors capable of providing supporting evidence for the shortlisting of contaminants.
- Utilise a rigorous decision-making process to prioritise CECs and generate a target shortlist.
Step 2 – Determine sampling locations and frequency, collection procedure, and analysis methodology
- Consider available sampling points, and the strengths and limitations in obtaining samples from each.
- Select a frequency with which target CECs will be quantified.
- Select a sampling methodology in consideration of the strengths and limitations of available sampling methods, as appropriate to the target CECs.
Step 1 of Phase 1 – Develop a target CEC list
There is an obvious need to shortlist target contaminants for economy within the CEC screening program. As a preliminary step, a broad review of literature was undertaken to identify what ‘factors’ could be used to provide supporting evidence either for, or against the short-listing of a contaminant.
Peer-reviewed articles from scientific databases such as ScienceDirect were retrieved describing different CEC class concentrations in wastewaters. International databases were also explored during the review. This included the NORMAN Suspect Database, a product of “an independent and highly recognized network of reference laboratories, research centres, and related organizations for the monitoring of contaminants of emerging concern” [15]. Such databases compile a plethora of information on CECs, including ecotoxicological endpoints and contaminant properties.
Local technical reports were also examined, including the NESP Report (2015) compiled by the Centre for Tropical Water and Aquatic Ecosystem Research (TropWATER). This report, titled ‘Identification, impacts, and prioritization of emerging contaminants present in the GBR and Torres Strait marine environments’, was a significant motivator in the evolution of the CBSTP CEC project, being the first to assess the qualitative risk of different CECs classes (i.e. pharmaceuticals versus pesticides) to the local marine environment.
The advice of industry professionals, academics, regulated substances lists, etc. were also considered during the review.
It became apparent during the review that there was no shortage of CEC suspect lists nor CECs to investigate, but rather a lack of prioritisation processes which focus specifically on those found in municipal STPs which may pose an ecological risk to receiving environments. Many CEC prioritisation approaches developed to date were designed to inform chemical regulation and are described extensively elsewhere [16, 17].
Usually each approach is based on one or more of the following underlying principles:
- CECs of high production volume (HPV) (the basis for chemical screening in the European Union, United States and Canada);
- Being of a persistent, bio-accumulative and or toxic (PBT) nature (the basis of the US Environmental Protection Agency’s PBT profiler [18]) or;
- Occurrence-based CEC prioritisation (e.g. exploiting historical chemical detections in surface waters) [19].
The Water Research Foundation has also developed a Bayesian framework for the Prioritization of Research into Constituents of Concern [20]. The web-based tool assists water authorities to determine from their own perspective which CECs require further research (e.g. considering whether contaminants pose unique challenges to the organisation).
In contrast, the decision-making process described here intends to capture those CECs considered most likely to pose a risk to the receiving environment.
Whilst each existing prioritisation approach holds merit and offers practical guidance to assessors of potential CEC risk, individually they may present oversight and bias in the context of wastewater discharge.
It would be easy, for example, to limit analysis to only regulated CECs, or alternatively those suggestive of aquatic toxicity at low environmental concentrations. However, this bias may manifest in the exclusion of CECs for which ecotoxicological data is limited, or perhaps whose “lower” toxicity is offset by high effluent concentrations.
From the literature review, factors considered valuable in providing supporting evidence for, or against the short-listing of a contaminant specific to STP discharges were collated.
These are presented under seven headings in Figure 4. This figure illustrates that the process of developing a target CEC list can be multi-dimensional. Whilst aspects of many factors are interrelated, it is important to note that the effort involved in compiling available data for each varies significantly.
Also unique to each factor is the degree of confidence in the datasets and likelihood that they will be representative of conditions at CBSTP. In consideration of these qualities, key strengths and limitations of each factor are also presented in this figure and are explored in greater detail in the discussion.
Our literature review provided an extensive initial list of 600 CECs which considered a comprehensive range of CEC classes. Supporting evidence from each of the seven factors [A to G] given in Figure 3 were then evaluated in order to filter, and thereby reduce, the number of CECs within each category.
The intent is to quantify an array of CECs from different chemical classes in CBSTP wastewaters simultaneously. Hence the need to target representative compounds within each class.
To demonstrate this process, supporting evidence for two CECs within a single CEC class are evaluated in the discussion section of this paper.
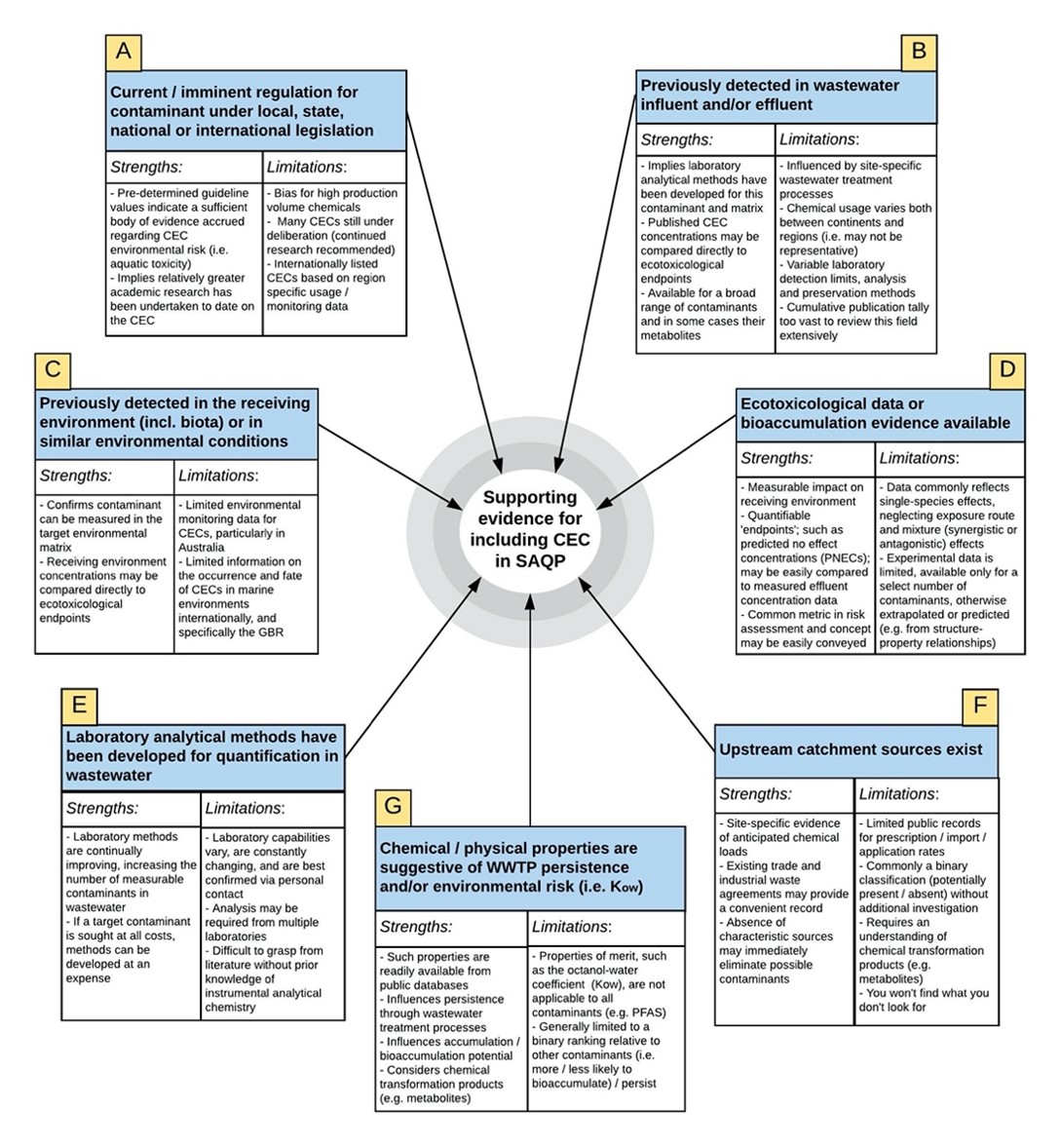 Figure 4: Decision-making factors which may contribute evidence for, or against the inclusion of a contaminant in the target shortlist (Step 1 of Phase 1)
Figure 4: Decision-making factors which may contribute evidence for, or against the inclusion of a contaminant in the target shortlist (Step 1 of Phase 1)
Step 2 of Phase 1 – Determine sampling locations and frequency, collection procedure, and analysis methodology
Selection of the most appropriate sampling locations at CBSTP is the second crucial step in SAQP development. For both practicality and resource economy, limiting the number of sampling locations for the preliminary screening was desirable.
Prior to selection, the strengths and weaknesses of each sampling location were considered in the context of the project objectives. Considerations were primarily focussed on the anticipated data representativeness and the foundational value that sampling from locations may offer in refining later receiving environmental monitoring programs.
Obtaining a sufficiently representative sample of CBSTP influent and effluent will be valuable to quantify, and subsequently prioritise, CECs at this site. Since the inherent daily, weekly and
seasonal variability in municipal influents and fluctuating STP process conditions cannot be avoided, at this stage in the overall project their impacts on measured CEC concentrations need to at least be considered.
Personal care products, some pharmaceuticals and pesticides, exhibit seasonal loadings dependent on their application; for example, insect repellent N,N-Diethyl-meta-toluamide (DEET) concentrations and many UV filters such as benzophenone-3 (BZ3) have been variously reported to increase significantly in summer [21, 22]. Thus, both an economical and practical method should be selected to provide insight into this variability to inform later investigations.
Ensuring CEC stability in the collected sample as much as practicable was also considered with the use of sample preservatives. This included devising a strategy for sample storage and transport.
Phase 2 – Undertake target CECs analysis
The analytical component of this project was achieved through cooperation with several laboratories.
The expertise of the Queensland Alliance for Environmental Health Sciences (QAEHS) laboratory (Brisbane) was applied to the analysis of pharmaceuticals, personal care products, pesticides, polyaromatic hydrocarbons, and organophosphorus flame retardants.
The Townsville Laboratory Services (TLS) analysed for metals, some pesticides, and PFAS. At a later date, Eurofins Environment Testing Australia (Eurofins) was engaged to quantify some of the many remaining antibiotics and PCP CECs of interest.
Whilst not the primary focus of this paper, the analytical methods (extraction and instrumental) adopted for CEC analysis have been described in Supplementary Data Tables.
Phase 3 – Review values of selected CECs
Following SAQP development and ensuing collection and analysis, a review of the results was required to assess the success or otherwise of the CEC selection methodology, as well as to explore the implications of these results on the future assessments of ecological risk. For example, the detectability of CECs above limits of detection (LOD), variability between sampling days and the effect of preservations methods were investigated.
Finally, CECs of particular interest will be discussed in greater detail with respect to the decision-making factors which justified their inclusion in the preliminary screening program.
Results and discussion
To explore these limitations and the target CEC decision-making process for CBSTP further, a comparison of CEC decision-making factors [A to G] (Figure 4) is presented for two contaminants within a single contaminant class, recalling that there is a need to choose compounds within each class to achieve analytical economy within the CEC screening program.
Comparison of respective data compiled on the decision-making factors are used to demonstrate which contaminant should be chosen for the target CEC list.
Applying decision-making factors to OPEs
Each of the seven decision-making factors [A to G] (Figure 4) are explored within this discussion as they apply to tris(butyl) phosphate (TNBP) and tris(2-chloroisopropyl) phosphate (TCIPP), just two of many organophosphate esters (OPEs) that could potentially be investigated as part of the CBSTP preliminary CEC screening program.
OPEs are widely used as plasticisers and flame retardants in an array of commercial products [23]. Their widespread use is likely to be reflected in the CEC influent profile of most municipal catchments, including CBSTP’s [factor F].
As HPV chemicals, they have been reported in an extensive range of environmental compartments from indoor air [24] to rainwater [25]. OPFRs including TCIPP have even been detected in chlorinated swimming pools, albeit at concentrations several orders of magnitude below commonly applied health risk benchmarks [26].
Their quantification in wastewaters is commonly achieved using gas chromatography–mass spectrometry (GC-MS), a well-established analytical method [factor E] [23, 24].
The octanol-water coefficient (Kow) amongst other parameters is frequently used in reviewing CEC environmental fate, as it describes the relationship between the fat and water solubility of a substance. A higher Kow indicates an increased tendency to partition to organic matter and animal lipids, and thus bioaccumulate.
Comparatively, TCIPP is more soluble in water at ambient temperatures [27], and TNBP presents the higher Kow of the two compounds (4.00 versus 2.59). Since both are <4.5 Kow bio-accumulative potential for both OPEs is low.
Neither TNBP nor TCIPP are regulated in Australia, and as such, toxicant guideline values are not provided within the Australian and New Zealand Guidelines for Fresh and Marine Water Quality [28] [factor A].
However, as a Priority Existing Chemical, TCIPP has been subject to preliminary assessment by the government’s National Industrial Chemicals Notification and Assessment Scheme (NICNAS) for TCIPP, identifying it as slightly toxic to aquatic organisms at all trophic levels but with prevailing data gaps in its subchronic or chronic reproductive toxicity [29].
Both TNBP and TCIPP have been measured in wastewater influent and effluent [factor B]. An EU-wide monitoring survey of 90 STPs revealed average concentrations of TCIPP (1231 ng/L) an order of magnitude higher than TNBP (260 ng/L).
Closer to home, a study by O’Brien, Thai [30] reported zero detections of TNBP in samples taken from eleven Australian STPs. In comparison, TCIPP was detected in all samples, ranging from 0.5-4.1 µg/L.
Looking to the NORMAN Network Chemical Occurrence Database, it reports tens of thousands of incidents of TCIPP and TNBP in surface waters [factor C], but with minimal seawater or marine water representation (<0.5%).
Applying the decision-making factors and in reflection of the above evidence, TCIPP was prioritised over TNBP, and consequently analysed as part of the preliminary screening program. This was primarily on account of the likelihood of detection in effluent and its status as a Priority Existing Chemical.
This process of prioritising and filtering CECs was repeated for the following contaminant classes: pesticides, flame retardants, polyaromatic hydrocarbons and alkyl phenols, personal care products, illicit drugs (and opiates), and pharmaceuticals.
The total number of target CECs analysed was reduced to 150 from a starting count of >600. Some variations from this target list were made in reflection of laboratory resourcing limitations.
Further, wherever additional contaminant analysis or laboratory-standard suites were readily or cheaply available (i.e. for PFAS and pesticides), these suites were adopted in full. A total of 301 CECs were therefore analysed.
Adopted sampling locations
Cleveland Bay is a tropical embayment of 325 sq km, located inshore of the central section of the Great Barrier Reef [31]. While the potential ecological risk to this environment is the ultimate project focus, undertaking preliminary CEC screening adjacent to the CBSTP effluent outfall presents numerous limitations.
In short, CECs may be readily diluted, transformed, adsorbed, and transported in the receiving environment from the point of discharge. This complexity can be overcome with extensive monitoring of all relevant matrices (water, sediment, biota, etc.) in the receiving environment.
The influence of surrounding anthropogenic activities to Cleveland Bay should also be considered in the ultimate risk assessment. These include port operations (north-west) and runoff from the Townsville State Development Area (TSDA) via Sandfly Creek, which discharges adjacent the CBSTP outfall.
It was considered that measuring CECs in the receiving environment would be better served once the chemical profile of the treated effluent was characterised for target CECs. This would reduce the number of sampling locations and matrices (e.g. sediments and surface waters) required.
Limiting CEC quantification to effluent, however, ignores the potential incidence of bypass releases, which are specified in CBSTP’s licence. Subject only to inlet coarse screening, bypassed flows may contain considerably higher concentrations of CECs than indicated by the effluent. Influent concentrations are not only a valuable indication of worst-case contaminant loads during bypass events; they also provide useful data on treatment process removal.
Observing removal of CEC concentrations after wastewater treatment may assist in identifying other contaminant degradation products or precursors, which may not have been included in the original target CEC list.
Sampling locations for CBPP’s preliminary effluent screening program were therefore limited to influent and effluent.
Adopted sample collection procedures
There are three primary methods generally available for the collection of effluent: grab, composite and passive sampling.
Grab sampling refers to the direct collection of a sample at a single point in time. A significant limitation of these samples is their inability to capture time-dependant variations in contaminant concentrations. Accounting for this variability is particularly relevant to STP discharges of CECs.
Composite samples allow for the collection of multiple individual samples obtained at a pre-determined rate (such as time or flow-weighted). This is typically performed over a 24-hour period. Composite sampling therefore represents the preferred sampling methodology for the CBSTP preliminary CEC screening program.
Moreover, many of the logistical issues associated with composite sampling at CBSTP are avoided because there are flow-weighted autosamplers on both influent and effluent streams. However, these do not completely eradicate technical issues. The installation of the autosamplers on horizontal pipes, for example, can result in inflated amounts of settled pipe material being collected.
Clogging of the autosampler nozzle drawing, or other calibration issues are also common. Careful monitoring of autosamplers by operators and thorough documentation of such incidences is recommended for quality control.
Passive sampling devices record in-situ measurements by their suspension in a waterbody, accumulating CECs to the device over time. The composition of the sampling device is specific to the analyte of interest and delivers only a semi-quantitative result. They are an alternative sampling tool also used to overcome short-term fluctuations in CEC concentrations by integrating CEC levels over extended sampling periods.
Despite the added benefit of potentially ‘amplifying’ target CECs that would otherwise be reported below detection limits with grab or composite sampling, passive samplers are more suited to sampling the receiving environment.
Once again, given the variability associated with quantifying CECs in the receiving environment, identifying the chemical profile of the treated effluent was considered a preceding step to the deployment of passive samplers.
Sampling methods adopted for CBPP’s preliminary effluent screening program were therefore comprised of composite (autosampler) samples on the influent and effluent.
Unfortunately, literature dedicated to the best-practice preservation methods for many CECs is lacking. When undertaking the analysis of a large array of chemicals, it is beneficial to reduce the number of sampling containers for practicality. Samples may need to be sent large distances for analysis.
For example, QAEHS laboratory (Brisbane) completed most of the analysis for pharmaceuticals, personal care products. Amber glass containers, for example, can break if not filled and stored correctly when attempting to freeze and transport.
High-density polyethylene (HDPE) bottles with unlined plastic caps, which are employed for a wide variety of analysis (including PFAS) were chosen as the containers for both influent and effluent.
To stabilise and preserve the analytes, acidification of the collected sample was applied. Hydrochloric acid can reduce sample pH to <2, minimising the degree of biological activity in combination with frozen storage temperatures (-18°C) [32]. As acidification has been shown to increase the concentrations of some compounds [32], an unpreserved sample was also collected for comparison.
Containers with sodium metabisulfite (SMBS) were also used to reduce any residual chlorine in the samples. Residual chlorine has been shown, to degrade endocrine disrupting chemicals [33]. Autosamplers may be adjusted to collect greater sample volumes to allow comparison of preservation methods.
Field blanks were prepared at CBSTP during the preliminary screening program in the same manner as the sample but applying ultra-pure MilliQ water. This was used to quantify the potential contamination during the collection procedure and potentially identify trace contamination problems associated with the sample containers and preservation. The sample was collected and stored in an identical manner to the primary samples.
Duplicate samples are valuable to measure precision in the collection process. Due to the high cost of analysis for many CECs, duplicate analysis was only conducted on a few CEC classes (e.g. PFAS, whose analysis at TLS was cost effective).
Irrespectively, the erraticism of contaminants profiles over the 7-day preliminary sampling period was expected to produce variability either equal to, or in exceedance of that which would be attained with daily duplication.
To provide sufficient volume for the analysis of over 100 CECs at QAEHS, the composite influent and effluent were collected every day for 7 days, triplicating each sample to assess the three preservation methods (nil, acidified, and dosed with SMBS).For analyses requiring large sample volumes in the laboratory (i.e. herbicides, pharmaceuticals and PPCPs), the daily samples were pooled to give a weekly composite. Samples were frozen, and shipped to QAEHS (Brisbane) at program conclusion.
Additional HDPE bottles (no preservative) were collected for local analysis of some CECs such as metals and metalloids, and PFAS at local Townsville Laboratory Services (TLS).
To quantify some of the many remaining CECs not determined from the primary sampling event (e.g. antibiotics), nil preservative amber glass containers were submitted to Eurofins during a secondary sampling round.
Analytical results
The results of the preliminary screening program are presented here to allow informed comment on the suitability and effectiveness of the decision-making framework.
It should be noted that validation of this process cannot be obtained from this study alone but provides a different perspective on the alternatives available to the water industry for screening CECs in STPs.
The primary sampling campaign was undertaken at CBSTP from 1-8 August 2019, and secondary on 9 June 2020. Courtesy of QAEHS, TLS, and Eurofins, a total of 301 CECs were successfully analysed in the laboratory.
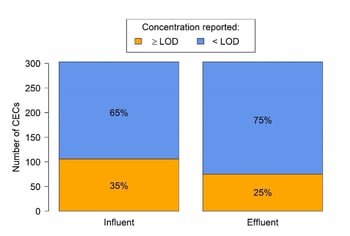
Figure 5 illustrates that most of the analysed CECs (62%) were below the laboratory limits of detection (LOD; generally ranging from 1-500 ng/L) in influent samples. Moreover, only a few of the CECs detected in the influent were subsequently removed to an extent such that their effluent concentrations were reported below LOD (11% of the total).
The concept of CEC ubiquity and persistence through STPs at measurable concentrations is therefore supported for CBSTP, with 76 compounds detected in the effluent.
In colloquial terms, if CEC concentrations occur at measurable concentrations entering STPs, then measurable concentrations will generally leave it. This is due in large part to improved analytical capabilities, but also the ineffectual removal of many CECs from STPs.
Turning now to the magnitude of CEC results, the highest influent concentration for any analyte was reported for the illicit drug 3,4-methylenedioxymethamphetamine (MDMA) (300 µg/L), followed by paracetamol (220 µg/L).
The highest reported effluent concentration was that of the anti-epileptic pharmaceutical carbamazepine (1.85 µg/L), followed by the antidepressant Venlafaxine (1.5 µg/L).
Similar concentrations for these pharmaceuticals, and other detected CECs such as pesticides, have been reported in the Cairns tertiary STPs which employs a submerged membrane filtration process [34], highlighting the importance of reviewing local literature on CEC occurrence with comparable treatment processes where available [factor B].
As described in the methodology, 24-hr composite influent and effluent samples were collected every day for 7 days and analysed where possible after factoring in the required analysis volumes.
The greatest daily variability of analyte concentration was reported for benzoylecgonine, the primary cocaine metabolite (203%, relative to the average result). Likewise, MDMA variability was also relatively high (163%). Most illicit drugs in fact had their peak influent concentrations in the Monday morning sample, suggesting an increased consumption during the weekend. Such temporal trends in illicit substances has been reported elsewhere [35, 36].
The implications of sampling and preservation methods have not been widely explored for many contaminant classes investigated here. It appears that sample preservation method (nil, acidified and SMBS dosed) did not significantly influence the results. That is, the variability between preservation methods was generally equal to or below that obtained in duplicate samples.
If this experiment were to be repeated, resources would be better placed refining (and potentially expanding) the CEC target list.
To provide an efficient snapshot of the environmental relevance of CEC concentrations measured in CBSTP effluent, a well-established hazard quotient (HQ) framework was adopted. The framework presents a quick and simplistic method for the non-expert in ecotoxicity and may assist in refining the scope of subsequent CEC investigations.
The HQ is calculated by dividing the measured environmental concentration in CBSTP effluent by the lowest predicted no effect concentration (PNEC) for the analyte (derived from experimental data and Quantitative Structure-Activity Relationships (QSAR) models) [12]. The indicative risk is then determined by a CEC’s HQ score.
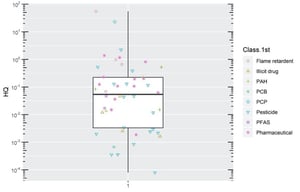 Figure 6: Hazard quotient (HQ) calculation for CECs detected in Cleveland Bay STP effluent calculated using the lowest reported predicted no effect concentration-PNEC (courtesy of the NORMAN Network, 2020)
Figure 6: Hazard quotient (HQ) calculation for CECs detected in Cleveland Bay STP effluent calculated using the lowest reported predicted no effect concentration-PNEC (courtesy of the NORMAN Network, 2020)
Figure 6 presents the HQs obtained for the different chemical classes whose effluent concentrations were reported above LOD. Using this metric, we conclude that most CECs present a low risk to the receiving environment (i.e. HQ<1).
Only five CECs reported a HQ above 1, comprising organophosphate flame retardant tris(2-chloroisopropyl) phosphate (HQ=55) [“high risk”], personal care product and fragrance galaxolide (HQ = 23) [“high risk”]
(noting analyte specific assumptions described in Supplementary Data Tables), pesticide terbutryn (HQ=2.2) [“low risk”], pharmaceutical and drug metabolite cotinine (HQ=1.4) [“low risk”], and antibiotic azithromycin (HQ=1.2) [“low risk”].
Considering the potential incidence of bypass releases, the HQ calculation was repeated for influent concentrations. The risk profile is understandably higher for influent than effluent, as more CECs present with a HQ>1 (11 CECs). Nevertheless, this remains a small fraction of the overall number investigated.
Interestingly, all five CECs with HQs above 1 have very different upstream catchment sources [factor F], require different analytical methods for quantification [factor E], and exhibit varying physiochemical properties [factor G]. Regarding detections in the receiving environment
[factor C], there is a lack of data for their occurrence in Cleveland Bay.
The implications of analytical detection limits [factor E] must be considered here, with 18% of CECs analysed presenting (lowest) PNECs below their respective LODs. In absence of a reported effluent concentration for these contaminants therefore, their relative risk remains inconclusive.
Furthermore, PNECs (lowest) were only available from pre-existing databases for 87% of the CECs analysed [37], reaffirming that the simple HQ metric is hampered by the availability of ecotoxicological information [factor D].
Given these results, the likelihood of obtaining detectable CEC measurements in receiving waters with grab samples are low for most CECs (after accounting for the additional dilution effects incurred at the mouth of CBSTP’s outfall).
Conversely, environmentally relevant concentrations of CECs are most likely to occur in receiving waters during low-tide conditions, when CBSTP discharges directly onto Cleveland Bay’s mudflats.
However, employing passive samplers in the water column, or focussing on the accumulative and/or bioaccumulative pathways for CECs may provide a more holistic perspective on their environmental persistence and ecological risk.
Of the five CECs reporting HQs above 1, azithromycin and galaxolide present the greatest bio-accumulative potential for aquatic organisms [38].
Conclusion
This paper has taken a considered approach to the development and execution of a SAQP for the screening of CECs in Cleveland Bay STP wastewaters.
The methods presented attempt to draw attention to the finer details of undertaking a CEC screening program in wastewaters, and the common oversights and limitations of investigating a potentially vast array of CECs.
This study demonstrated the benefit of applying a broad array of decision-making factors to a preliminary investigation, to indicatively assess the apparent environmental risk of CECs across a variety of CEC classes. As such, the indicative environmental risk determined from established methods was essentially non-existent for all the contaminant classes investigated.
Conversely, CEC classes suggestive of potential risk came from a variety of CEC classes (flame retardants, pharmaceuticals and pesticides). This demonstrates the importance of considering broader CEC classes when pursuing assessments of ecological risk for STP discharges.
This paper, including the decision-making process for determining target CECs within the various contaminant classes (demonstrated for two OPEs), offers guidance to other water authorities concerned with the quantification of ecological risk posed by CECs discharged in effluent.
While the outcomes in this work focus on the conditions of CBSTP, the decision-making factors for shortlisting CECs for a preliminary CEC screening are applicable to other STPs. These provide supporting evidence for or against the assessment of CECs specific to municipal wastewaters over others, irrespective of the wastewater treatment level or the type of receiving environment for the effluent (e.g. fresh versus marine waters).
More importantly, following the preliminary screening program, TCC’s knowledge of CECs discharging to Cleveland Bay has been improved. This knowledge will shape subsequent sampling and analysis strategies for CECs in the region, including receiving environmental analysis for CECs in Cleveland Bay.
Acknowledgements
Sincere thanks are extended to the laboratories who conducted the CEC analysis for the preliminary CEC investigation, comprising Professor Jochen Mueller’s team from the Queensland Alliance for Environmental Health Sciences (QAEHS), Townsville Laboratory Services (TLS) and the team at Eurofins Environment Testing Australia (Eurofins) in Brisbane for their provision of invaluable support and technical guidance in the analysis of CECs.
The authors also extend thanks to Townsville City Council for sponsoring the CBSTP CEC research project, and whose employees have contributed time and resources to the successful undertaking of the preliminary effluent screening program.
About the author
Laura Kuskopf | Laura Kuskopf is a chemical engineer with GHD who is undertaking a part-time PhD at JCU. In collaboration with TCC, her research is investigating the potential ecological risks of emerging contaminants in wastewater effluent discharged to Cleveland Bay.
Dr Madoc Sheehan | Dr Madoc Sheehan is an Associate Professor at JCU and Teaching Coordinator for the Chemical Engineering degree programme. He holds broad systems analysis and modelling experience and has applied these skills across energy, food and water sectors.
Anna Whelan | Anna Whelan is a process engineer with over 15 years’ experience in North Queensland’s wastewater industry. Her current role focuses on the process and environmental compliance of Townsville’s six wastewater treatment plants and the substantial wastewater pumping network.
References
Simon, J.A., The unprecedented rate of developments concerning PFAS issues. Remediation Journal, 2019. 29(2): p. 3-5.
AUSTRALIA, H.O.E. and N. Zealand, PFAS National Environmental Management Plan Version 2.0. The National Chemicals Working Group of the Heads of EPAs Australia and New Zealand (HEPA), 2020.
Chen, H. and M. Zhang, Occurrence and removal of antibiotic resistance genes in municipal wastewater and rural domestic sewage treatment systems in eastern China. Environment International, 2013. 55: p. 9-14.
Czekalski, N., E.G. Diez, and H. Burgmann, Wastewater as a point source of antibiotic-resitance genes in the sediment of a freshwater lake. International Society for Microbial Ecology, 2014. 8: p. 1381-1390.
Preen, A., Dugongs, boats, dolphins and turtles in the Townsville-Cardwell region and recommendations for a boat traffic management plan for the Hinchinbrook Dugong Protection Area. 2000: Great Barrier Reef Marine Park Authority.
Vijayasarathy, S., et al., Multi-residue screening of non-polar hazardous chemicals in green turtle blood from different foraging regions of the Great Barrier Reef. Science of The Total Environment, 2019. 652: p. 862-868.
Bartell, S.M., Ecological Risk Assessment, in Encyclopedia of Ecology, S.E. Jørgensen and B.D. Fath, Editors. 2008, Academic Press: Oxford. p. 1097-1101.
Norton, S.B., et al., A framework for ecological risk assessment at the EPA. Environmental toxicology and chemistry, 1992. 11(12): p. 1663-1672.
Carpenter, R.A., Risk assessment. Impact Assessment, 1995. 13(2): p. 153-187.
Rizzo, L., et al., Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Science of The Total Environment, 2019. 655: p. 986-1008.
Petrie, B., R. Barden, and B. Kasprzyk-Hordern, A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Research, 2015. 72: p. 3-27.
Kroon, F., et al., Identification, impacts, and prioritization of emerging contaminants present in the GBR and Torres Strait marine environments, in National Environmental Science Programme. 2015, Reef and Rainforest Research Centre Limited: Cairns. p. 138.
Kroon, F.J., et al., Sources, presence and potential effects of contaminants of emerging concern in the marine environments of the Great Barrier Reef and Torres Strait, Australia. Science of The Total Environment, 2020. 719: p. 135140.
Fabbri, E. and S. Franzellitti, Human pharmaceuticals in the marine environment: focus on exposure and biological effects in animal species. Environmental toxicology and chemistry, 2016. 35(4): p. 799-812.
Dulio, V., et al., Emerging pollutants in the EU: 10 years of NORMAN in support of environmental policies and regulations. Environmental Sciences Europe, 2018. 30(1): p. 5.
Guillén, D., et al., Prioritization of chemicals in the aquatic environment based on risk assessment: Analytical, modeling and regulatory perspective. Science of The Total Environment, 2012. 440: p. 236-252.
Arnot, J.A. and D. Mackay, Policies for chemical hazard and risk priority setting: can persistence, bioaccumulation, toxicity, and quantity information be combined? 2008, ACS Publications.
Clarke, R. and E. Cummins, Evaluation of 'classic' and emerging contaminants resulting from the application of biosolids to agricultural lands: a review. 2014. 21: p. 492-513.
Diamond, J.M., et al., Prioritizing contaminants of emerging concern for ecological screening assessments. Environmental toxicology and chemistry, 2011. 30(11): p. 2385-2394.
Khan, S.J., et al., Decision-Making Framework for the Prioritization of Research into Constituents of Concern. 2019.
Fairbairn, D., et al., Sources and transport of contaminants of emerging concern: A two-year study of occurrence and spatiotemporal variation in a mixed land use watershed. Science of the Total Environment, 2016. 551-552: p. 605-613.
Giokas, D.L., A. Salvador, and A. Chisvert, UV filters: From sunscreens to human body and the environment. TrAC Trends in Analytical Chemistry, 2007. 26(5): p. 360-374.
Reemtsma, T., et al., Organophosphorus flame retardants and plasticizers in water and air I. Occurrence and fate. TrAC Trends in Analytical Chemistry, 2008. 27(9): p. 727-737.
Marklund, A., B. Andersson, and P. Haglund, Organophosphorus flame retardants and plasticizers in Swedish sewage treatment plants. Environmental science & technology, 2005. 39(19): p. 7423-7429.
Regnery, J. and W. Püttmann, Organophosphorus Flame Retardants and Plasticizers in Rain and Snow from Middle Germany. CLEAN – Soil, Air, Water, 2009. 37(4‐5): p. 334-342.
Teo, T.L.L., H.M. Coleman, and S.J. Khan, Presence and select determinants of organophosphate flame retardants in public swimming pools. Science of The Total Environment, 2016. 569-570: p. 469-475.
US Department of Health Human Services, Hazardous substances data bank (HSDB, online database). National Toxicology Information Program, National Library of Medicine, Bethesda, MD, 2010.
ANZECC, A., Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, Canberra, 2018: p. 1-103.
NINCAS, Triphosphates: Priority Existing Chemical Assessment Report No. 17. 2001.
O’Brien, J.W., et al., Wastewater analysis of Census day samples to investigate per capita input of organophosphorus flame retardants and plasticizers into wastewater. Chemosphere, 2015. 138: p. 328-334.
- Anderson, C.B., R.M Carter, J.Cruz, G.Doherty, G.Ericson, E.Evansillidge, D.Haynes, A.Hesse, G.B.Jones, P.Larcombe, J.Lough, K. Neil, A.Orphin, A.J.Reichelt-Brushett, P.Ridd, M.Roche, V.Veitch, Cleveland Bay, Townsville, Queensland Australia: Status Report - 2002. Cleveland Bay Consortium, 2002.
EPA, U., Stability of Pharmaceuticals, Personal Care Products, Steroids, and Hormones in Aqueous Samples, POTW Effluents, and Biosolids. 2010.
Saggioro, E.M., et al., Endocrine Disruptor Degradation by UV/Chlorine and the Impact of Their Removal on Estrogenic Activity and Toxicity. International Journal of Photoenergy, 2019. 2019.
O’Brien, D., et al., Barron River Pesticide Monitoring and Cairns WWTP WQ Assessment. 2014, Centre for Tropical Water and Aquatic Ecosystem Research (TropWATER): Cairns. p. 48.
Thiebault, T., et al., Temporal dynamics of human-excreted pollutants in wastewater treatment plant influents: Toward a better knowledge of mass load fluctuations. Science of The Total Environment, 2017. 596-597: p. 246-255.
Tscharke, B.J., et al., Temporal trends in drug use in Adelaide, South Australia by wastewater analysis. Science of the Total Environment, 2016. 565: p. 384-391.
(NORMAN), N.o.r.l.r.c.a.r.o.f.m.o.e.e.s., Substance Database. 2020.
Information, N.C.f.B. PubChem Compound Summary for CID 447043, Azithromycin & CID 91497, Galaxolide. 2020 [cited 10/08/2020]; Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Azithromycin; https://pubchem.ncbi.nlm.nih.gov/compound/Galaxolide.